Objectives:
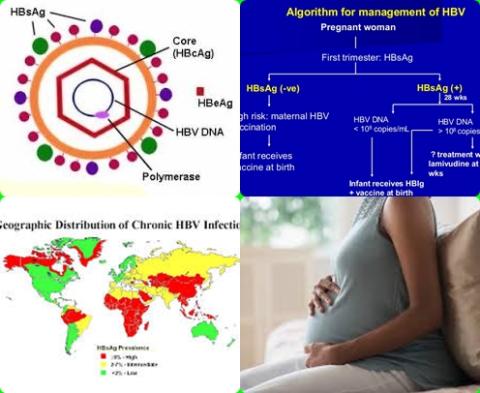
Prevention of mother-to-child transmission (MTCT) of hepatitis B virus (HBV) involves neonatal immunoprophylaxis, with a birth dose of hepatitis B vaccine and immune globulin, and provision of peripartum antiviral prophylaxis in highly viraemic women. However, access to assays to quantify HBV DNA levels remains inadequate in resource-poor settings. Therefore, this review article has been conducted.
The aims of this review article are to identify the HBV DNA threshold for MTCT, to assess the sensitivity and specificity of hepatitis B e antigen (HBeAg) testing to identify pregnant women with HBV DNA levels above this threshold and to predict MTCT of HBV infection on the basis of HBeAg testing?
Study design:
This review article included 66 studies.
Results and conclusions:
The investigators found the risk of mother-to-child transmission (MTCT) despite infant immunoprophylaxis was negligible [0.04%, 95% CI = 0.00 to 0.25] below a maternal HBV DNA level of 5.30 log10 IU/mL (200,000 IU/mL) and increased above this threshold.
The investigators found the pooled sensitivity of HBeAg testing to identify HBV DNA levels of 5.30 log10 IU/mL or greater in pregnant women was 88.2% [95% CI = 83.9 to 91.5] and pooled specificity was 92.6% [95% CI = 90.0 to 94.5].
The investigators found the pooled sensitivity of HBeAg testing in predicting MTCT of HBV infection despite infant immunoprophylaxis was 99.5% [95% CI = 91.7-100] and pooled specificity was 62.2% [95% CI = 55.2 to 68.7].
The investigators concluded maternal HBV DNA of 5.30 log10 IU/mL or greater appears to be the optimal threshold for MTCT of HBV infection despite infant immunoprophylaxis. HBeAg is accurate to identify women with HBV DNA levels above this threshold and has high sensitivity to predict cases of immunoprophylaxis failure. In areas where HBV DNA assays are unavailable, HBeAg can be used as an alternative to assess eligibility for antiviral prophylaxis.
Original title:
Accuracy of HBeAg to identify pregnant women at risk of transmitting hepatitis B virus to their neonates: a systematic review and meta-analysis by Boucheron P, Lu Y, […], Shimakawa Y.
Link:
https://pubmed.ncbi.nlm.nih.gov/32805201/
Additional information of El Mondo:
Find more information/studies on vaccination and malnutrition right here.